Synolostats
Your Partner for Pharmaceutical CMC
and Manufacturing Statistics
Leverage Statistics Across the Process and Analytical Lifecycle

Process Design
- QbD
- Design of Experiments (DOE)
- Design Space
- Specification Derivation and Acceptance Criteria
- Shelf-Life
- Predictive Modelling
- Assay Validation
- Method Robustness
Process Performance Qualification (PPQ/PV)
- Sampling Plans (intra-and inter-batch)
- Initial Capability Analysis
- Evidence of Process Control and Reproducibility
- Predictive Modelling
- Variance Component Analysis
- Comparability
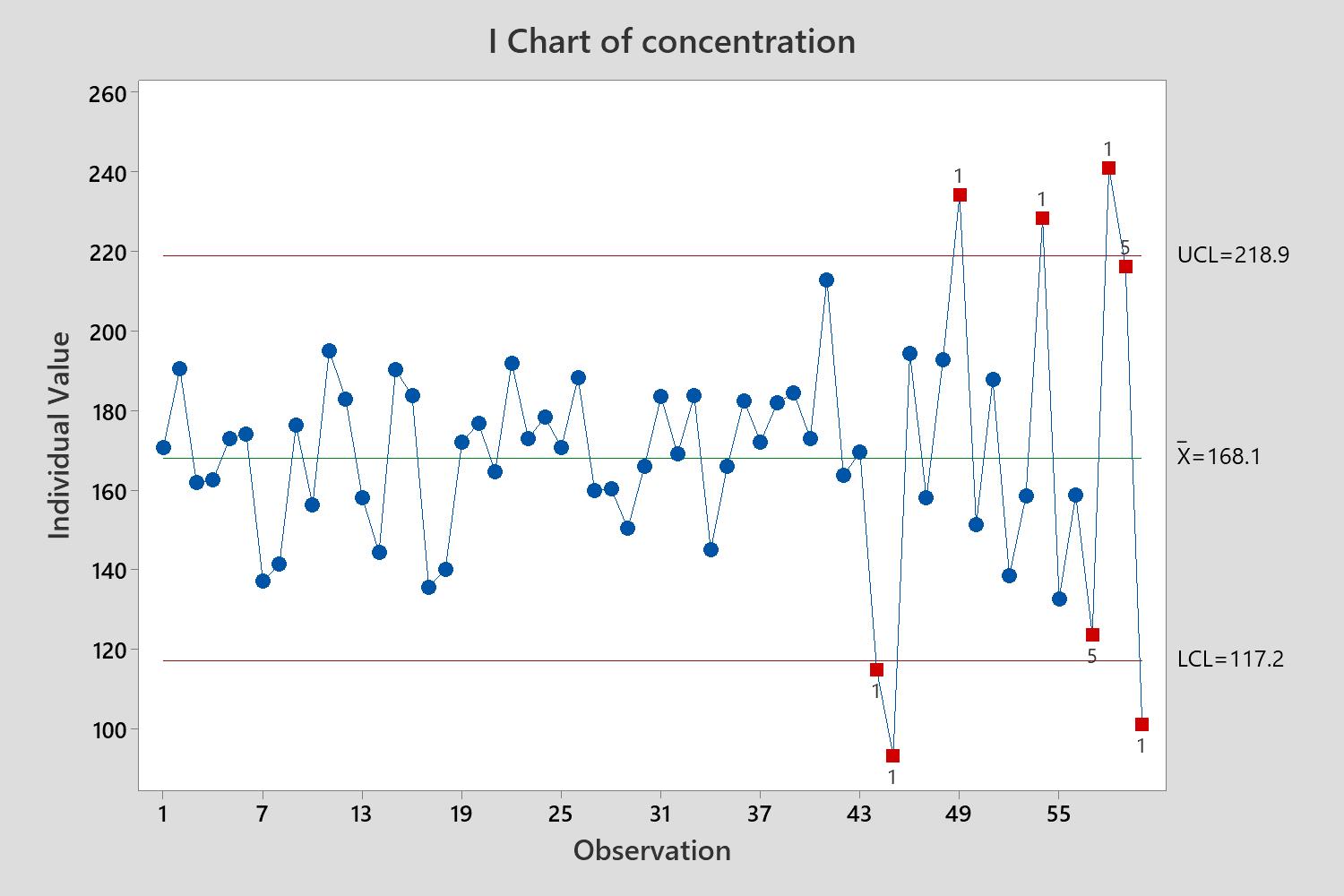
Ongoing Monitoring (CPV/OPV)
- Design of a CPV program
- Enhanced Sampling Plans
- Statistical Process Control
- Process Capability
- Root Cause Analysis
- Stability Monitoring
- Data Visualization
Training Topics/Courses
Build statistical thinking into your organization
- Quality by Design
- Design of Experiments
- Statistical Process Control for CPV/OPV
- Data Visualization and Problem Solving
- Sampling and Acceptance Criteria
- Stability Monitoring

Our Philosophy
Our passion and goals are the same as yours
We partner with you to leverage the powerful combination of science and statistics to accelerate timelines, design and develop robust processes, and assure ongoing quality to patients and consumersWe deliver sensible solutions
Our name reflects our holistic approach (Synolo is Greek for total.) We seek the most practical solution that is statistically appropriate AND maximizes value. We leverage our broad knowledge of manufacturing, business, and regulatory processes to find an optimal solution that incorporates relevant requirements of each.